7 July 2025
Starpharma presents additional clinical data for DEP® cabazitaxel in prostate cancer (ASX Announcement)
- Starpharma presented promising anti-tumour activity data in prostate cancer at the European Society of Medical Oncology (ESMO) Congress on Sunday
- The new data presented shows DEP® cabazitaxel had a number of key advantages compared to published data for conventional cabazitaxel, including superior efficacy measured by longer progression-free survival (PFS)[1] and lower incidence of key side effects, in a heavily pre-treated patient cohort
- DEP® cabazitaxel showed multiple potential benefits for patients with metastatic Castration-Resistant Prostate Cancer (mCRPC), including:
- Median PFS was 3.9 months[2], more than 30% longer than the 2.9 months[3] reported for standard cabazitaxel
- Lower incidence of severe (Grade 3 or 4) treatment related adverse events (TRAEs) for DEP® cabazitaxel of 7.5%2, compared to published data for standard cabazitaxel of 39.7%3
- No severe hypersensitivity reactions or requirement for patients to have steroid pre-medication, in contrast to standard cabazitaxel
Melbourne, Australia; 12 September 2022: Starpharma (ASX: SPL, OTCQX: SPHRY) announces additional results from the mCRPC cohort of its Phase 2 DEP® cabazitaxel trial, following completion of dosing in this cohort. Treatment with DEP® cabazitaxel showed a number of key advantages compared to published data for conventional cabazitaxel, including superior efficacy, as measured by longer PFS, and a lower incidence of key side effects, despite this patient cohort being relatively more heavily pre-treated.
The new data for DEP® cabazitaxel was presented in a scientific poster at the ESMO 2022 Congress in Paris, France, by Principal Investigator, Professor Robert Jones of the Velindre Cancer Centre in Wales. The poster is available on www.starpharma.com.
View ESMO Poster (1MB).
Starpharma CEO, Dr Jackie Fairley, commented:
“Starpharma is very pleased to report this additional encouraging data at the ESMO Congress for DEP® cabazitaxel in heavily pre-treated, late-stage prostate cancer patients, including median progression-free survival for the first time, having now completed dosing in this cohort. These latest results show DEP® cabazitaxel achieved both a longer duration of progression-free survival and fewer severe side effects compared to published data on Jevtana®, illustrating the potential for DEP® cabazitaxel to provide better outcomes for mCRPC patients.
“We are deeply appreciative of the cancer patients who have participated in this trial, along with the contribution of the clinical investigators involved in the study.”
Trial and Patient Cohort Overview
Twenty-five patients with metastatic castration-resistant prostate cancer were enrolled in this cohort across five trial sites in the UK and Australia. Trial participants received an intravenous infusion of DEP® cabazitaxel every 21 days, repeated for up to 12 cycles. The median time on study was 18.4 weeks.
All patients enrolled in this cohort had already been heavily pre-treated before entering the study, having previously received an average of 4 other cancer treatment types, in addition many have also had surgery and radiation. On average, patients enrolled in this study had already received more than 70 cycles/months of other treatments. Notably, 96% of patients in this trial cohort had also previously received related chemotherapies (taxanes), including docetaxel and/or conventional cabazitaxel (Jevtana®). This level of pre-treatment is important to note because patients with this high level of prior cancer treatment would not be expected to respond as well to further similar therapies.
Summary of interim results
- Highly encouraging anti-tumour activity for DEP® cabazitaxel, including a radiological partial response (PR) for more than 45 weeks, and stable or improved secondary metastatic bone disease for up to 45 weeks;
- Median progression-free survival (PFS) of 3.9 months2 for DEP® cabazitaxel which is more than 30% longer than published PFS data for standard cabazitaxel (2.9 months3) (see below and Table 1) at the same dose;
- 100% of evaluable DEP® cabazitaxel patients achieved a response in at least 1 measure of efficacy (soft tissue disease [stable disease (SD) or PR], prostate specific antigen (PSA), and/or bone disease);
- 90% of DEP® cabazitaxel patients evaluable for a PSA response achieved a reduction in PSA, and 52% achieved a PSA reduction of 50% or more from baseline;
- 83% of DEP® cabazitaxel patients evaluable for secondary bone disease experienced an improvement or no progression;
- 68% of DEP® cabazitaxel patients evaluable for 2 or 3 efficacy measures achieved a response for all evaluable measures (soft tissue disease [SD or PR], PSA, and bone disease);
- No DEP® cabazitaxel patients required routine steroid pre-medication or daily oral steroid and only 2 patients required prophylactic G-CSF[4]; and
- DEP® cabazitaxel was generally well-tolerated, with TRAEs similar to those observed with standard cabazitaxel (Jevtana®).
Progression-free survival
Notably, the median PFS observed in evaluable mCRPC patients treated with DEP® cabazitaxel was longer than published data on Jevtana®. Patients treated with DEP® cabazitaxel at the recommended Phase 2 dose (20 mg/m2) achieved a composite median PFS of at least 3.9 months2. This is a more than 30% improvement in median PFS than what has been reported for patients treated with Jevtana® (2.9 months3) at the same dose (20 mg/m2). Even at a higher dose of Jevtana® (25 mg/m2) published data show patients treated with Jevtana® achieved a median PFS of 3.5 months3 in one study and 2.8 months[5] in another, so DEP® cabazitaxel (20 mg/m2) median PFS was longer.
Table 1. Longer PFS (median) observed in patients treated with DEP® cabazitaxel compared to published data on Jevtana®
|
DEP® cabazitaxel (20 mg/m2) (N=25) |
Jevtana®3 (20 mg/m2) (N=598*) |
Jevtana®3 (25 mg/m2) (N=602*) |
Jevtana®5 (25 mg/m2) (N=378*) |
|
3.9 months |
2.9 months |
3.5 months |
2.8 months |
|
* Intent-to-treat (ITT) populations |
|||
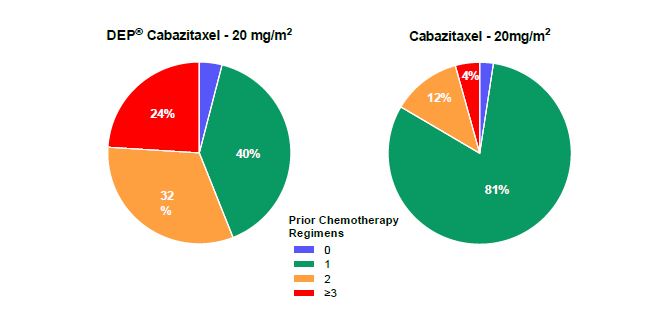
These very encouraging findings for DEP® cabazitaxel in this study were observed despite many patients being at an increased risk of neutropenic complications, due to their age (mean = 73 years) and large number of prior chemotherapy regimens. More than half the DEP® cabazitaxel patients (56%2) had received at least two prior chemotherapy regimens, whereas only 16%3 of patients from published Jevtana® data had received this level of prior treatment (Figure 1). DEP® cabazitaxel patients received an average of 4 other cancer treatments, and more than 70 cycles/months of treatment before entry into the study.
Figure 1. Number of prior chemotherapy regimens for patients in the Phase 2 mCRPC cohort vs trial of conventional cabazitaxel (Jevtana®)2
Adverse Events
In this heavily pre-treated DEP® cabazitaxel cohort, TRAEs were generally mild to moderate, and all have also been reported for Jevtana®. In addition, the incidence of Grade 3 and 4 TRAEs in patients treated with DEP® cabazitaxel was only 7.5%2, substantially lower than published reports for Jevtana® (39.7%3) at the same dose (20 mg/m2) (see Table 2).
Notably, the incidence of Grade 3 and 4 neutropenia was 16.0%2 in this cohort of patients treated with DEP® cabazitaxel, less than half of the 41.8%3 reported for patients treated with Jevtana®. A lower incidence of problematic severe bone marrow toxicities was also observed in patients treated with DEP® cabazitaxel compared to published data on Jevtana®3. Notably, and despite the older age of this cohort, secondary prophylactic use of G-CSF was only required by 2 patients.
Table 2. Lower incidence of Grade 3 and 4 TRAEs observed in patients treated with DEP® cabazitaxel compared to published data on Jevtana®
|
DEP® cabazitaxel |
Jevtana®3 (20 mg/m2) (N=580†) |
Jevtana®3 (25 mg/m2) (N=595†) |
|
7.5% |
39.7% |
54.5% |
|
† Safety populations (received at least 1 dose) |
||
Table 3. Lower incidence of severe bone marrow toxicities in patients treated with DEP® cabazitaxel compared to published data on Jevtana®
|
Bone Marrow Toxicity |
DEP® cabazitaxel (20 mg/m2) (N=25) |
Jevtana®3 |
|
Neutropenia* ≥ grade 3 |
16.0% |
41.8% |
|
Febrile neutropenia ≥ grade 3 |
0% |
2.1% |
|
Thrombocytopenia* ≥ grade 3 |
0% |
2.6% |
|
Neutropenic infection / sepsis |
0% |
2.1% |
|
* Lab detected neutropenia or thrombocytopenia, regardless of whether or not event was reported as an adverse event † Safety population (received at least 1 dose) |
||
Patients with mCRPC in this DEP® cabazitaxel study have now completed dosing. These new results, together with the previously reported interim findings (see ASX announcement dated 25 November 2021), indicate an improved and favourable efficacy and safety profile of DEP® cabazitaxel compared to published data on Jevtana®.
In addition to the positive Phase 2 clinical data reported here for DEP® cabazitaxel in prostate cancer, encouraging efficacy signals, including multiple partial responses, have been observed in other cancer types, often in heavily pre-treated patients. These cancer types include platinum resistant ovarian cancer, and other relapsed and refractory cancers, including oesophageal squamous cell carcinoma and gastro-oesophageal junction adenocarcinoma. Starpharma is recruiting a number of additional patients with these tumour types into the trial as positive findings in these indications could expand the application of DEP® cabazitaxel and its market potential. Final results from the DEP® cabazitaxel trial will be reported following completion of dosing and analyses of all cohorts, however the data reported at the ESMO Congress will feed into ongoing licensing discussions.
DEP® cabazitaxel
Developed by Starpharma, DEP® cabazitaxel is a patented, dendrimer nanoparticle version of conventional cabazitaxel, which is marketed as Jevtana® and widely used in the treatment of prostate cancer. Unlike standard cabazitaxel, DEP® cabazitaxel is highly water soluble, does not contain toxic detergent-like excipients associated with anaphylaxis, and avoids the need for steroid pre-medication. In both preclinical and clinical studies, DEP® cabazitaxel has shown an improved side effect profile, notably markedly reduced bone marrow toxicity demonstrated by lower rates of severe neutropenia, thrombocytopenia, and severe anaemia, which are all reportedly experienced by a significant proportion of patients treated with Jevtana®.
Download ASX Announcement: Starpharma presents promising additional clinical data for DEP® cabazitaxel in prostate cancer
View ESMO Poster (1MB).
[1] Progression-free survival (PFS) is the length of time during and after the treatment of a disease, such as cancer, that a patient lives with the disease but it does not progress. For this study, PFS was defined as the time from start of treatment to the first of PSA or radiologic progression.
[2] Jones, RH, et al. Efficacy and Safety of Dendrimer-Enhanced (DEP®) Cabazitaxel (CTX-SPL9111) in Men with Metastatic Castration-Resistant Prostate Cancer (mCRPC) in a Phase 1/2 Trial, ESMO 2022 Congress, FPN 1403P.
[3] Eisenberger, M, et al. J Clin Oncol, 2017;35(28):3198-206.
[4] G-CSF: granulocyte-colony stimulating factor, is used as a therapy for myelosuppression. Myelosuppression is a condition in which bone marrow function is adversely impacted, resulting in fewer red blood cells, white blood cells, and platelets. It is a side effect of some cancer treatments.
[5] de Bono, JS, et al. Lancet, 2010;376(9747):1147-54.