19 September 2025
DEP® docetaxel and DEP® cabazitaxel outperform in human pancreatic cancer model
- DEP® cabazitaxel, both alone and in combination with gemcitabine, showed complete tumour regression and 100% survival in a human pancreatic cancer model
- DEP® docetaxel alone significantly outperformed standard treatments gemcitabine and/or Abraxane® and achieved 100% survival
- DEP® docetaxel when combined with gemcitabine showed complete tumour regression and 100% survival in this model
- The complete tumour regression induced by both DEP® products was despite the fact that standard pancreatic cancer treatments, gemcitabine and Abraxane® alone and in combination showed limited activity in this pancreatic cancer model
- DEP® docetaxel is currently in phase 2 trials both alone and in combination with nintedanib (Vargatef®) and DEP® cabazitaxel is in phase 1 / 2 trials - this data in pancreatic cancer will feed into the clinical development program and patient selection going forward
Melbourne, Australia; 16 November 2018: Starpharma (ASX: SPL, OTCQX: SPHRY) today announced that its proprietary DEP® docetaxel and DEP® cabazitaxel products, which are both currently in the clinic, showed significant efficacy and safety benefits over gemcitabine (Gemzar®) alone, Abraxane® (Nab-paclitaxel) alone and in combination, in a human pancreatic cancer model. These impressive efficacy results were despite the fact that these standard pancreatic cancer treatments, gemcitabine and/or Abraxane® showed limited activity in this model.
Starpharma’s DEP® cabazitaxel alone and in combination with gemcitabine, and DEP® docetaxel in combination with gemcitabine, resulted in complete tumour regression and 100% survival, significantly outperforming each standard treatment, gemcitabine and Abraxane® alone and in combination. DEP® docetaxel alone significantly outperformed both gemcitabine and Abraxane® in this pancreatic cancer model, in terms of both tumour regression and survival.
Pancreatic cancer is a leading cause of death among oncologic diseases, with a one-year relative survival rate of 20%, and a five-year survival rate of only 7%. Gemcitabine is commonly used both alone and in combination with Abraxane® in pancreatic cancer as a first line treatment. Annual sales of Abraxane® are approximately US$1.2 billion[1]. Gemcitabine, which is now generic, had peak sales prior to patent expiry of US$1.7 billion.
These impressive efficacy results in the human pancreatic cancer model (Figures 1 to 4) build on previously announced data for these two products in a number of other tumour types. DEP® docetaxel is in phase 2 trials, both alone and in combination with nintedanib (Vargatef®) while DEP® cabazitaxel is in phase 1 / 2 trials. This latest data in pancreatic cancer will feed into the clinical development program and patient selection going forward.
Dr Jackie Fairley, Starpharma CEO, commented: “These results for both DEP® docetaxel and DEP® cabazitaxel, showing complete tumour regression and 100% survival in this pancreatic cancer model are very impressive compared with the standard treatments of gemcitabine and/or Abraxane®. Given pancreatic cancer has one of the lowest survival rates, it remains an area of significant unmet medical need. These results are particularly interesting given we also observed stable disease for more than 20 weeks in a pancreatic cancer patient with DEP® docetaxel in our phase 1 trial.”
Study Results
In this preclinical human pancreatic cancer model, standard therapies gemcitabine and Abraxane® alone displayed limited tumour inhibition, with no tumour regression (Figure 1).
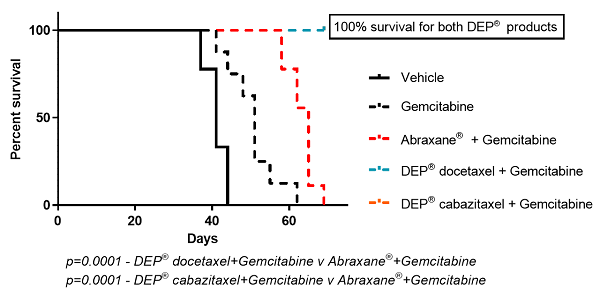
In contrast, DEP® cabazitaxel both alone and in combination with gemcitabine, resulted in complete tumour regression and 100% survival (Figures 1 to 4). Starpharma’s DEP® docetaxel alone was significantly more efficacious than gemcitabine or Abraxane® and also resulted in 100% survival, and in combination with gemcitabine demonstrated significant anti-cancer efficacy with complete tumour regression and 100% survival (Figures 1 to 4). These impressive efficacy results are despite the fact that this human pancreatic cancer model was poorly responsive to standard treatments, gemcitabine and/or Abraxane® (Figures 1 to 4).
Figure 1: Tumour volume vs time: DEP® docetaxel and DEP® cabazitaxel compared with gemcitabine and Abraxane® as monotherapies in a mouse xenograft (human pancreatic cancer model)
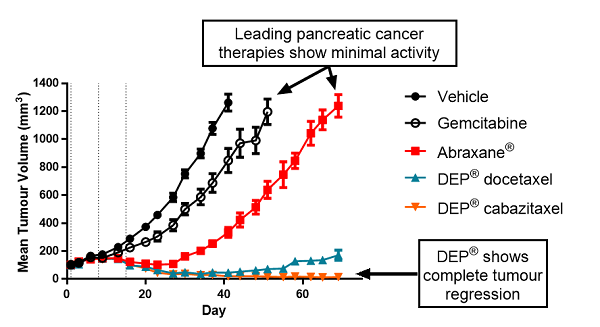
Figure 2: Tumour volume vs time: DEP® docetaxel and DEP® cabazitaxel in combination with gemcitabine compared with gemcitabine alone and in combination with Abraxane® in a mouse xenograft (human pancreatic cancer model)
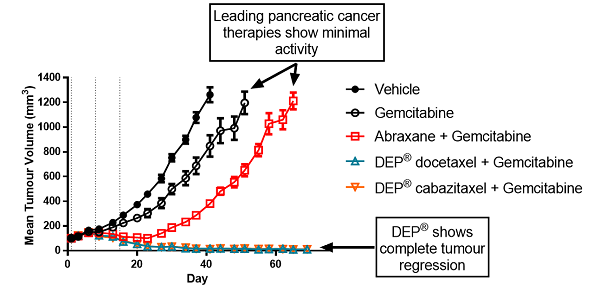
Figure 3: Kaplan Meier Survival Curve comparing DEP® docetaxel and DEP® cabazitaxel versus other groups
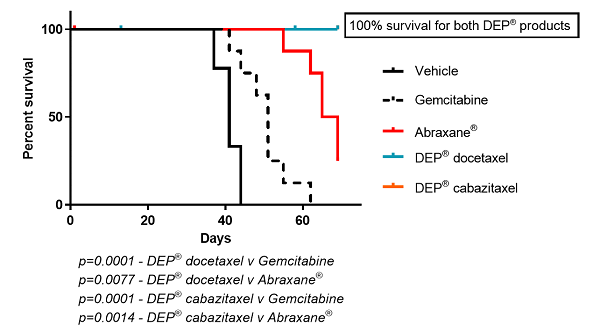
Figure 4: Kaplan Meier Survival Curve comparing DEP® docetaxel + gemcitabine and DEP® cabazitaxel + gemcitabine versus other groups
Study Methods
This preclinical mouse xenograft study was conducted for Starpharma by a leading international cancer research institution. The NOD/SCID-Gamma (NSG) mice were inoculated subcutaneously with CAPAN-1 human pancreatic ductal adenocarcinoma cell line (9 mice/group, respectively). Mice were dosed with saline vehicle, DEP® docetaxel[2], DEP® cabazitaxel2, gemcitabine (80mg/kg), Abraxane® (40mg/kg), gemcitabine + Abraxane® (80 and 40 mg/kg), DEP® docetaxel2 + gemcitabine (80mg/kg) or DEP® cabazitaxel2 + gemcitabine (80 mg/kg) on days 1, 8 and 15 (all drug groups). Tumour growth data were analysed in GraphPad Prism for ANOVA followed by Dunnett’s post-hoc test. The tumour volume data represent the mean ± standard error of the mean (SEM). Survival analysis was carried out using Kaplan-Meier survival curves and the Log-rank test. (Note: If error bars do not display on the graphs, they are shorter than the height of the symbol and not visible.)
Download ASX Announcement: DEP® docetaxel and DEP® cabazitaxel outperform in human pancreatic cancer model (PDF, 378kb)
[1] 2017 sales sourced from Medtrack database.
[2] Dose not disclosed pending intellectual property filings