21 August 2025
Successful VivaGel® Phase 3 results and NDA planned for rBV
- VivaGel® BV demonstrated statistically significant efficacy in two pivotal phase 3 trials
- VivaGel® BV consistently resulted in reduced rates of BV recurrence by the primary efficacy endpoint and five secondary efficacy measures, and delayed time to first recurrence
- VivaGel® BV resulted in sustained benefits 3 months after cessation of treatment
- The majority of women who used VivaGel® BV in both studies remained BV-recurrence-free during the 16-week treatment phase
- VivaGel® BV demonstrated excellent safety and tolerability, including very low rates of candidiasis
- These trial results strongly support marketing applications to US FDA and other regulators for rBV indication and add significant commercial value to VivaGel® BV
- FDA QIDP and Fast Track designations already granted for VivaGel® BV, providing significant commercial and regulatory advantages
- VivaGel® BV New Drug Application (NDA) is well-advanced for both BV indications (treatment and prevention of rBV)
- Phase 3 trial data significantly enhances the commercial opportunity for VivaGel® BV through the ongoing licensing process, facilitated by a global healthcare investment bank
Melbourne, Australia; 7 August 2017: Starpharma (ASX: SPL, OTCQX: SPHRY) today announced that its two phase 3 trials of VivaGel® BV for prevention of recurrent bacterial vaginosis (rBV) achieved their primary objective demonstrating statistically significant superiority compared to placebo in preventing rBV based on topline data.
Starpharma intends to submit a marketing application to the FDA for VivaGel® BV for prevention of rBV based on these positive results. There are currently no approved products for the prevention of rBV, which is a significant unmet medical need.
The two double-blind, randomised, placebo-controlled trials, SPL7013-017 (017 US trial) and SPL7013-018 (018 European trial), were identical in design and enrolled 1,223 women who had a history of rBV. A history of rBV was defined as at least three episodes of BV in the preceding 12 months (i.e. average of at least one recurrence every 16 weeks). Trial participants used either VivaGel® BV (1% SPL7013 Gel) or placebo gel on alternate days for 16 weeks. The 017 US trial was conducted at sites in the US, Puerto Rico, Canada and Mexico, and the 018 European trial was conducted at sites mainly in Europe but also included some sites in Thailand and the US.
The primary endpoint of both studies was BV recurrence at or by week 16 as diagnosed by clinical findings (i.e. presence of three out of four Amsel criteria). For the primary efficacy analyses, any patients who failed to attend the Week 16 visit were deemed to have recurred i.e. were imputed to failure (even if in reality they remained BV free), making this a very rigorous efficacy result.
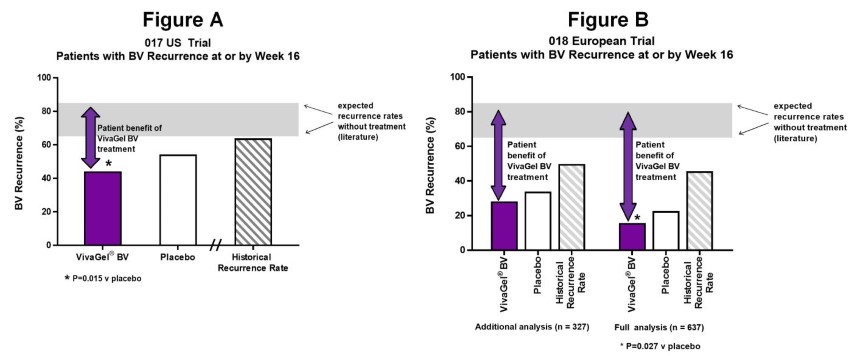
In the 017 US trial, the rate of BV recurrence at or by Week 16 (i.e. the primary endpoint) in the VivaGel® BV group was 44.2% (statistically significant versus placebo 54.3%, P=0.015, N=585) – see Figure A below. Actual BV recurrence rates, not imputing missing data to failure, were even lower at 34.9% for VivaGel® BV and 46.6% for placebo.
It has been observed in the literature that vaginally delivered placebos can have effect on BV, as was seen in both these trials. Therefore, in assessing the patient benefit of VivaGel® BV in this trial (apart from comparing to placebo) it is also useful to refer to expected rates of BV recurrence over a 16-week period without any intervention at all (i.e., placebo or active). Recurrence rates over 16-weeks in untreated rBV patients range between 65-85%[1] in the literature. In addition, a 16-week Historical Recurrence Rate (HRR) using the trial participants’ historical BV recurrences immediately prior to commencing the trial was estimated. This 16-week Historical Recurrence Rate for the trial participants in the 017 US trial was approximately 65%.
In the 018 European trial, the rate of BV recurrence at or by Week 16 in the VivaGel® BV group was just 15.7% (statistically significant versus placebo 22.6%, P=0.027, N=636) – see Figure B below. In comparison, the 16-week Historical Recurrence Rate (without intervention) for the 018 European trial participants was approximately 50%.
Given the rates of BV recurrence in the 018 European trial were lower than expected, and low compared with the 017 US trial, an investigation was conducted prior to data unblinding, and efficacy analyses (additional analysis) were also conducted on a modified subset population. This additional analysis excluded a number of sites in countries (e.g., Ukraine and Romania) where recurrence rates were lower than anticipated. In this additional analysis, the same pattern of benefit of reduced recurrence was also demonstrated for VivaGel® BV compared with placebo as for the full analysis, although due to the reduced sample size in this subset compared with the full analysis, the difference was not statistically significant (VivaGel® BV recurrence rate 28.2% versus placebo 33.9%, P=0.266, N=327).
In addition to the individual trial results reported above, when the data from both trials is combined, statistically significant differences between the rates of BV recurrence in the VivaGel® BV group versus placebo are also clearly demonstrated (017 US trial plus 018 European trial full analysis P=0.002, 017 US trial plus 018 European trial additional analysis P=0.014).
Further to the compelling benefits of VivaGel® BV in the primary endpoint, VivaGel® BV demonstrated statistically significant benefits compared with placebo in five secondary efficacy endpoints, including:
- Time to recurrence of BV (017 US trial P=0.007; 018 European trial full analysis P=0.009, additional analysis P=0.055);
- Reduced recurrence of patient reported symptoms of vaginal odour and/or discharge (017 US trial P<0.001; 018 European trial full analysis P=0.019, additional analysis P=0.032);
- Reduced recurrence of BV by Nugent score of 7-10 (017 US trial P=0.012; 018 European trial full analysis P=0.002, additional analysis P=0.016);
- Reduced recurrence of BV by clinical findings (i.e. 3 out of 4 Amsel criteria) and Nugent score greater than or equal to 4 (017 US trial P=0.008; 018 European trial full analysis P=0.014, additional analysis P=0.045); and
- Reduced recurrence of individual Amsel criteria as assessed by clinicians, including discharge (017 US trial P=0.015; 018 European trial full analysis P=0.011, additional analysis P=0.012), positive whiff test (017 US trial P=0.082; 018 European trial full analysis P=0.010, additional analysis P=0.022) and clue cells (017 US trial P=0.014; 018 European trial full analysis P=0.001, additional analysis P=0.008).
VivaGel® BV also resulted in sustained benefits well beyond cessation of treatment. Reduced recurrence of BV by the primary and secondary efficacy endpoints (including discharge, odour and clinical findings) were observed not only during the 16-week treatment period, but were also sustained during the 12-week follow-up period off-treatment.
Starpharma greatly appreciates the time and effort of the many women who volunteered for participation, along with the excellent support of clinicians and healthcare professionals in these trials.
Download the full announcement Successful VivaGel Phase 3 results and NDA planned for rBV (pdf file, 424kb) for detailed results and the trial design (Appendix 1), and a glossary of terms.
Results commentary
Dr Jackie Fairley, Starpharma Chief Executive Officer said: “We are delighted to report these successful phase 3 trial results, in which VivaGel® BV has demonstrated compelling efficacy in all six primary and secondary efficacy measures. Our NDA for VivaGel® BV for both treatment and rBV is well-advanced, and we’ll be using these data to complete the clinical package for submission to the FDA and other regulatory authorities.”
“There’s a desperate need for new therapeutic options for BV, a serious condition that affects nearly 1 in 3 women globally. The fact that VivaGel® BV is not a conventional antibiotic and specifically targets BV bacteria, makes it a particularly appealing solution for patients. It also represents a highly attractive commercial proposition especially given it will be first in class for the prevention of rBV. VivaGel® BV has potential to gain a significant share of this market, which is estimated to be in excess of US$1 billion per annum globally,” added Dr Fairley.
“Antibiotic resistance is a major issue globally and VivaGel® BV offers an alternative to conventional antibiotic therapies for BV. We know that patients and clinicians are very attracted to the non-antibiotic nature of the product, its novel mechanism of action on biofilm, and the fact that it is not absorbed into the bloodstream contributing to its excellent safety and tolerability profiles,” concluded Dr Fairley.
Expert comments on VivaGel® BV and the trial results
Professor Jane Schwebke: “I have many patients who have recurrent BV and suffer from frequent episodes of the condition. It’s very frustrating for these women and also for me as their physician that there are no effective therapies available, so the development of an efficacious BV preventative will be highly valued. In light of the importance of biofilm in the pathogenesis of BV, a therapy such as VivaGel® BV that disrupts biofilm would be most welcome for both the treatment and prevention of the condition.”
Dr Jane Schwebke, MD is a Professor of Medicine in the Infectious Disease Division at the University of Alabama at Birmingham and Consultant for the Jefferson County Department of Health STD clinic, Key Opinion Leader, world authority in bacterial vaginosis and Principal Investigator in the 017 US trial.
Professor George Kinghorn: “BV is very common and often a persistent condition. Management of recurrent BV is problematic and there is a significant need for products that work differently to existing antibiotics and are suitable for use longer term.
In both phase 3 VivaGel® BV studies of rBV, the majority of VivaGel® BV treated women remained recurrence free during the 16-week treatment period. In addition, the product demonstrated consistent benefits in terms of improvements in patient symptoms and in objective clinician and bacteriologic assessments in those treated with VivaGel® BV as compared with placebo. The treatment was well tolerated and there was a very low rate of vaginal candidiasis. As a clinician, I’m impressed with the trial data for VivaGel® BV for rBV and believe that it will offer a new management tool for this very troublesome condition.”
Dr George Kinghorn is an international expert in bacterial vaginosis having worked in the field for 40 years, most recently as Consultant Physician and Professor in the Department of Genitourinary Medicine, Royal Hallamshire Hospital, in Sheffield, UK.
Dr Philip McCloud: “The results for VivaGel® BV across these pivotal trials are strong. In the analyses VivaGel® BV demonstrated consistent and statistically significant benefits compared to placebo across multiple efficacy measures.”
Dr Philip McCloud, International Biostatistics & Data Management Specialist, Director & Principal Statistician of McCloud Consulting Group. Dr Philip McCloud has 40 years’ experience as an applied statistician at the top levels of the Pharmaceutical Industry, Government and Academia, overseeing the provision of expert statistical consulting and clinical data management. He was the biostatistician for these VivaGel® BV phase 3 trials.
Next Steps
These trial results strongly support marketing applications to the US FDA and other regulators for the BV prevention indication and add significant commercial value to VivaGel® BV.
The FDA new drug application (NDA) for VivaGel® BV for both treatment and rBV is well-advanced and data from the trials reported today will be incorporated to complete the clinical package. The NDA will be submitted to the FDA as soon as practicable with the initial sections of the rolling submission due for lodgement shortly. Throughout the preparation of the NDA, Starpharma continues to leverage the QIDP designation and Fast Track status granted by the FDA for VivaGel® BV. These designations carry significant benefits for regulatory approval and commercialisation, including increased dialogue with the FDA, priority regulatory review and an additional five years of market exclusivity. Starpharma also has a Special Protocol Agreement in place from the FDA for VivaGel® BV which provides binding FDA agreement on the phase 3 trial design.
In addition, the data from these trials will also be submitted to other regulatory authorities including in Europe, to expand the indications for VivaGel® BV to include rBV.
Negotiations are continuing with a number of parties for regional and global commercial rights to VivaGel® BV. These trial results confirm the product’s utility in both treatment and rBV and will have a significant positive impact on value. Starpharma has recently appointed a leading global healthcare investment bank to support the competitive process and for finalising commercial arrangements with potential partners.
About Bacterial Vaginosis (BV)
Bacterial vaginosis is the most common cause of vaginal infection for women of childbearing age, and affects around 30% of women in the US. It is a highly recurrent condition with 50-60% of sufferers having it recurrently. BV is caused by an imbalance of naturally occurring bacterial flora (the usual bacteria found in a woman's vagina). Smoking, the use of some hygiene products and several other risk factors are linked to a higher risk of developing BV. If left untreated, BV can cause a range of serious medical problems including pelvic inflammatory disease, infertility, premature delivery and miscarriage, low birth weights and uterine infection.BV also increases a woman’s chance of acquiring HIV and other sexually transmitted infections and increases the likelihood that a woman will infect her partner with these conditions.
About VivaGel® BV
VivaGel® BV is a water based gel for topical treatment and rapid relief of bacterial vaginosis (BV). It is based on Starpharma’s SPL7013, astodrimer sodium, a proprietary dendrimer that blocks certain bacteria involved in BV and also has potent antiviral activity against certain viruses (HIV, HSV, HPV, Zika).
The VivaGel® BV treatment product, which is already approved in Europe, targets an area of significant unmet medical need in a high-value market (est. US$750M) and has been licensed to Aspen Pharmacare with preparations underway for launch. A second VivaGel® BV product recently completed phase 3 clinical development for the prevention of recurrent BV which is another high value market (est. US$1B) for which there are currently no clinically approved products.
Download ASX Announcement: Successful VivaGel Phase 3 results and NDA planned for rBV (pdf file, 424kb)
[1] According to key opinion leader (KOL) estimates and literature reports:
- Larsson, 1992; Boris et al, 1997; Vutyavanich et al, 1993