31 August 2025
DEP® irinotecan outperforms irinotecan in multiple cancer models
Melbourne, Australia; 6 June 2017: Starpharma (ASX: SPL, OTCQX: SPHRY) today announced that its proprietary DEP® irinotecan has demonstrated significantly better anti‑tumour activity and increased survival compared with irinotecan in a variety of human colon cancer models.
DEP® irinotecan is Starpharma’s dendrimer version of the already marketed major cancer drug, irinotecan, and one of several Starpharma internal DEP® candidates under development (others include DEP® docetaxel and DEP® cabazitaxel).
Irinotecan is primarily used to treat colorectal cancer, which, although one of the most common cancer types, remains an area of significant unmet need with few treatment options. Irinotecan was originally commercialised under the brand name Camptosar® and achieved peak sales of US$1.1 billion prior to losing patent exclusivity. Irinotecan has a US FDA “Black Box” warning for severe diarrhoea and myelosuppression (including neutropenia).
In Starpharma’s studies, a single treatment cycle of DEP® irinotecan administered on days 1, 8 and 15 significantly improved anti-tumour activity and enhanced survival compared to irinotecan (Camptosar®) in all cancer models tested. In the SW-620 colon cancer model, DEP® irinotecan resulted in complete tumour regression and 100% survival.
Following these impressive results for DEP® irinotecan, the Company is expediting its development and scaling up the drug for further pre-clinical studies prior to clinical trials.
Starpharma is presenting these results and other DEP® clinical programs in meetings with clinicians and partners at the American Society of Clinical Oncology (ASCO) Annual Meeting in Chicago this week. ASCO brings together more than 35,000 oncology professionals, including internationally recognised oncologists, and a wide range of global pharmaceutical companies.
Starpharma CEO, Dr Jackie Fairley, commented: “These impressive results for DEP® irinotecan are promising in their own right, and more so when coupled with the mounting data from both our internal and partnered DEP® programs, which consistently demonstrate reproducible benefits in efficacy and tolerability from our DEP® platform using a range of anti-cancer drugs. The optionality associated with our DEP® technology is enabling us to build a pipeline of enhanced drugs, such as DEP® irinotecan, and represents a very attractive commercial scenario for Starpharma.
“We are focused on rapidly building value across the DEP® portfolio and our recently commissioned internal scale-up facilities will allow us to manufacture DEP® irinotecan, as well other internal and partnered DEP® candidates, to accelerate their development,” concluded Dr Fairley.
DEP® irinotecan is one of several anti-cancer DEP® products being developed internally by Starpharma, along with DEP® docetaxel, which is currently in the advanced stages of phase 1 clinical trial and will soon enter phase 2, and DEP® cabazitaxel, which will enter clinical trials later this year. Starpharma also has a number of partnered DEP® programs, including multiple compounds in drug development with AstraZeneca and world-leading antibody-drug conjugate companies.
About Irinotecan
Conventional irinotecan is a pro-drug, which, following intravenous administration, needs to be converted in the liver to the active anti-cancer agent, known as SN-38. In contrast, Starpharma’s DEP® irinotecan incorporates the active irinotecan derivative (i.e., SN-38), avoiding the need for hepatic conversion. DEP® irinotecan is expected to accumulate preferentially in tumour tissue to exert its superior anti-tumour effect, as is seen with DEP® docetaxel and other DEP® conjugates (Diagram 1).
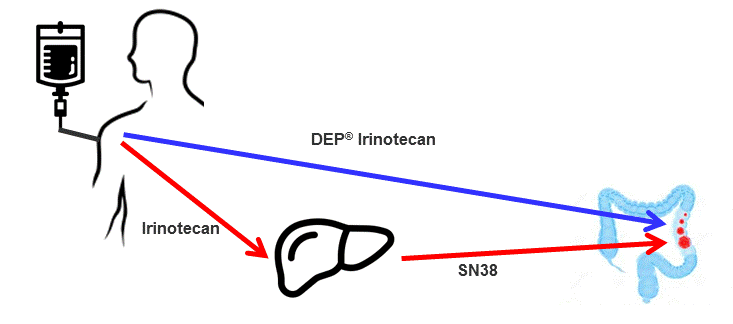
Diagram 1: Graphical representation of conversion of irinotecan to SN-38 by the liver, and the direct delivery of DEP® irinotecan to the tumour
Irinotecan is used as a component of first line therapy for the treatment of colorectal cancer (CRC), which is one of the most common cancers in the world, affecting more than 1 million individuals annually. CRC is the third most common cancer to affect both men and women, and the third-leading cause of cancer-related death, accounting for 8% of all annual cancer mortalities[1].
Study Methods and Results
Two xenograft studies (using HT-29 and SW-620 – human colon cancer cells) were conducted for Starpharma by an internationally recognized translational cancer group as part of a wider program of studies to assess DEP® conjugates. A xenograft study uses human cancer cells, which are then implanted in a mouse, and is a well‑established means of assessing efficacy of anti-cancer therapies.
Balb/c mice were inoculated subcutaneously with either the HT-29 or SW-620 cancer cell line (10 and 6 mice/group, respectively). Mice were dosed with saline (vehicle), DEP® irinotecan (25mg/kg) and irinotecan (90mg/kg) on days 1, 8 and 15 (all drug groups were dosed at the pre-determined maximum tolerated dose for each therapy). Tumour growth data were analysed in GraphPad Prism for ANOVA followed by Dunnett’s post-hoc test. The tumour volume data represent the mean ± standard error of the mean (SEM). Kaplan-Meier survival curves were analysed using the Log-rank (Mantel-Cox) test.
SW-620 Xenograft:
Complete tumour regression and 100% survival was observed in DEP® irinotecan treated animals bearing the colon cancer tumour cell line, SW-620. The tumour inhibition and survival effects of DEP® irinotecan were markedly improved compared with the irinotecan treated group and the differences were highly statistically significant (P<0.0001 and P<0.0045, respectively) (see Figures 1 and 2).
Figures 1 and 2
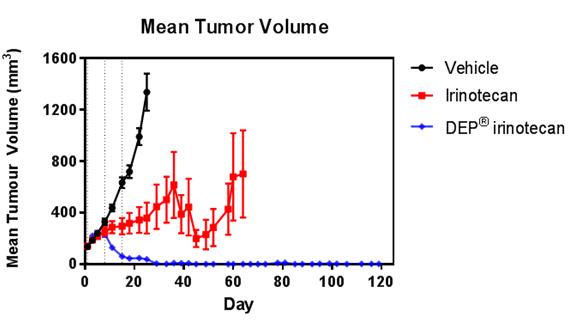
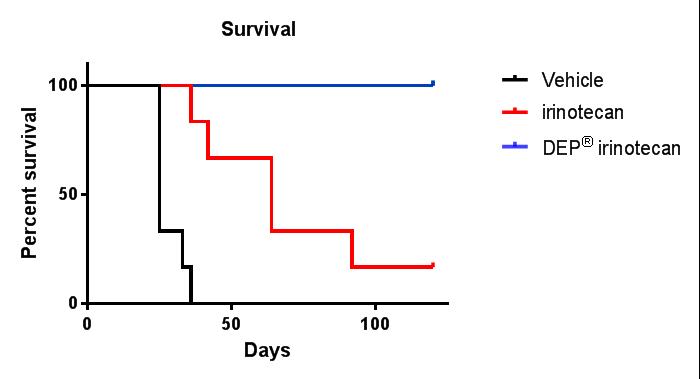
Figures 1 and 2: Efficacy (anti-tumour effect – mean tumour volume) and survival curves comparing DEP® irinotecan to irinotecan and saline (vehicle) control. DEP® irinotecan showed markedly enhanced efficacy and survival rates compared to irinotecan and the differences were highly statistically significant (P<0.0001 and P<0.0045, respectively).
In DEP® irinotecan-treated animals, complete tumour regression was seen as early as day 29 after the first dose and maintained to the end of the study at day 119 (Figure 3). In this model, there was no tumour regression seen with irinotecan (the anti-cancer effect was limited to a delay in tumour growth). DEP® irinotecan was well tolerated in the model.
Figure 3
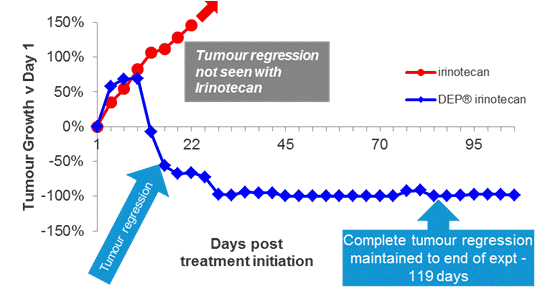
Figure 3: Tumour regression – SW-620 xenograft: DEP® irinotecan induced complete tumour regression in all mice tested, an effect that was sustained until the end of the study (day 119). This enhanced effect was highly statistically significant compared with the effect of irinotecan (P<0.0001). In contrast to treatment with DEP® irinotecan, tumour regression was not observed following treatment with irinotecan, and irinotecan only induced a delay in tumour growth compared with vehicle.
HT-29 Xenograft:
DEP® irinotecan was also shown to be very effective in this colon cancer (HT-29) tumour model, which typically responds poorly to irinotecan (Figures 4 and 5). DEP® irinotecan treatment resulted in an 11.8-fold improvement in survival compared with irinotecan (Table 1).
In this study, irinotecan did not achieve appreciable anti-cancer activity compared to saline, whereas DEP® irinotecan exhibited a significant anti-cancer effect (Figure 4). DEP® irinotecan was significantly more effective than irinotecan (P<0.0001) for both enhanced efficacy and survival (see Figures 4 and 5). DEP® irinotecan was also well tolerated in this model.
Figure 4
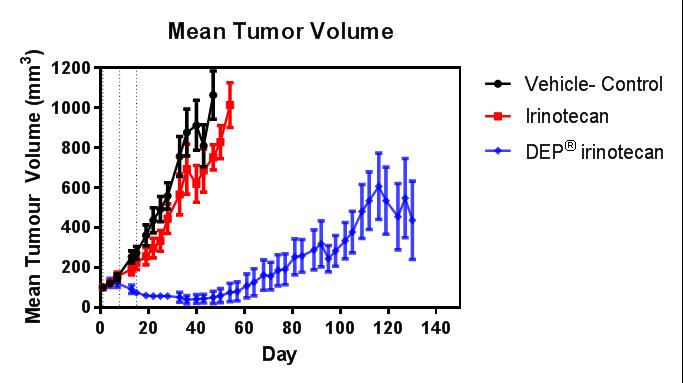 Figure 5
Figure 5
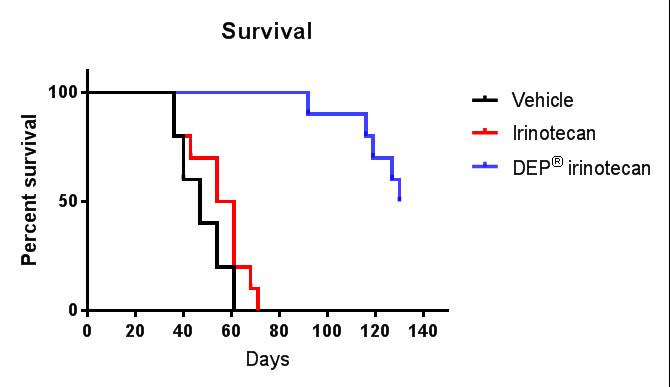
Figures 4 and 5: Efficacy (anti-tumour effect – mean tumour volume) and survival curves comparing DEP® irinotecan to irinotecan and vehicle control. DEP® irinotecan showed enhanced efficacy and survival rates compared with irinotecan and the differences were highly statistically significant (P<0.0001 and P<0.0001, respectively).
Table 1
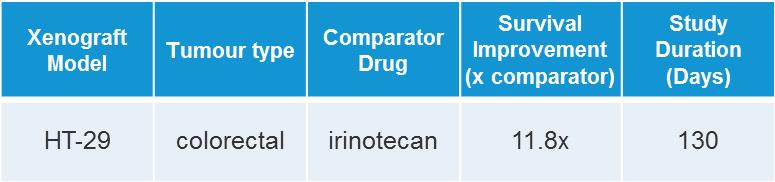
Table 1: The enhanced survival benefit seen in mice treated with DEP® irinotecan was an 11.8-fold improvement compared to that seen with irinotecan, with a 50% survival increase of 83 days over saline (vehicle). Irinotecan survival improvement was only 7 days more than that seen with vehicle.
The HT-29 cell-line is a cell-line in which irinotecan effectiveness is poor. Despite this, DEP® irinotecan was able to induce substantial tumour regression, which was not seen with irinotecan (Figure 6). A significantly enhanced tumour inhibition effect was seen with DEP® irinotecan compared with irinotecan (P<0.0001 – Dunnett’s post-hoc test). At day 36, tumour growth inhibition versus saline (vehicle) was only 21% for irinotecan and 95% for DEP® irinotecan.
This significant differential anti-tumour effect of the two agents demonstrates the potential for DEP® irinotecan to overcome the lack of responsiveness to irinotecan in this model.
Figure 6
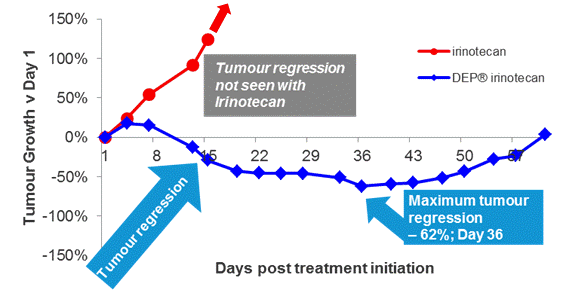
Figure 6: Tumour regression – HT-29 xenograft: DEP® irinotecan induced tumour regression in all mice tested, an effect that was observed until the end of the study (day 130). This enhanced effect was statistically significant compared with the effect of irinotecan (P<0.0001). In contrast to treatment with DEP® irinotecan, tumour regression was not observed following treatment with irinotecan, and irinotecan only induced a very short transient delay in tumour growth compared with vehicle.
[1] Datamonitor report – Colorectal cancer (Disease Coverage) – DMKC10877
Download ASX Announcement: DEP® irinotecan outperforms irinotecan in multiple cancer models ( pdf file, 349kb)